PREMs
Patient Reported Experiences Measurements
Objective
To develop a simple tool that allows to collect the experience of the transplanted pediatric patient and their families during the whole process. This tool will allow having information from the patients to implement improvements in the process that will impact the health and well being of the patients.
On the other hand, the Madrid Health System will become a reference, not only for the improvement in the health and well-being of patients, but also for the improvement in the health processes of the Madrid centers where they are implemented.
Methodology
To achieve the proposed objective, the following phases have been designed:
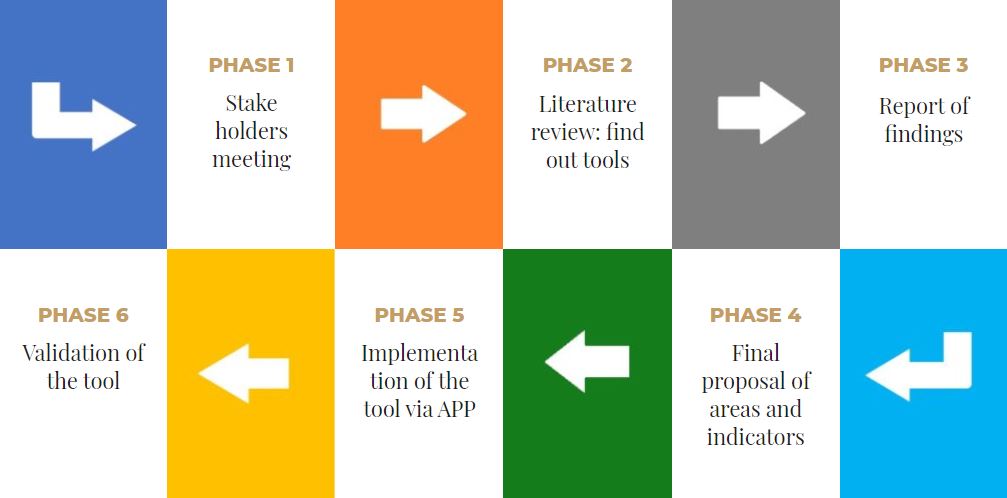
PHASE 1
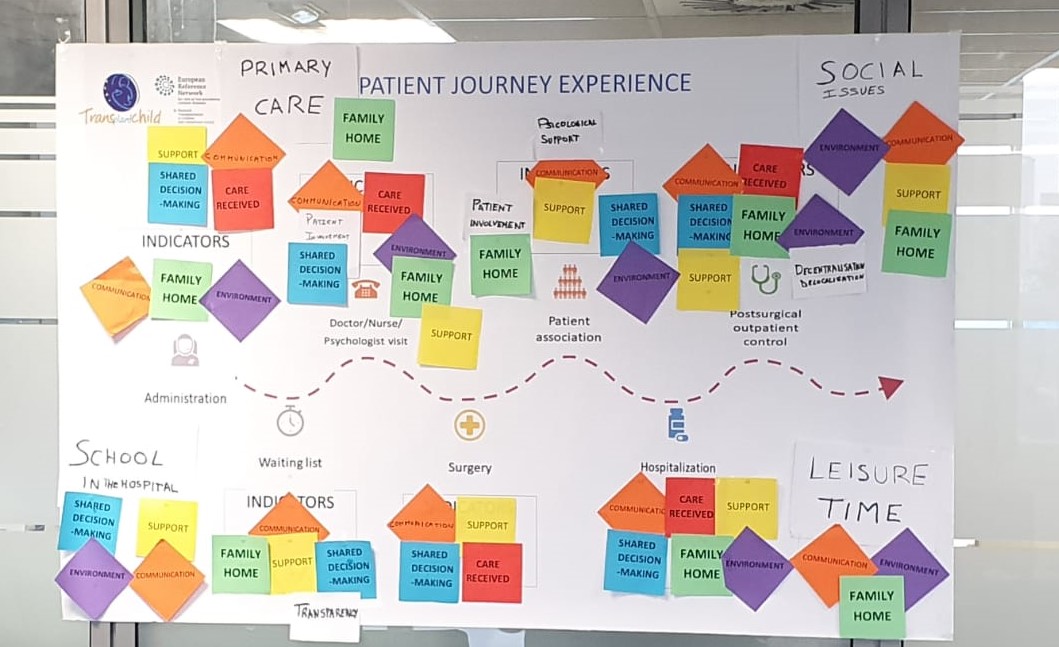
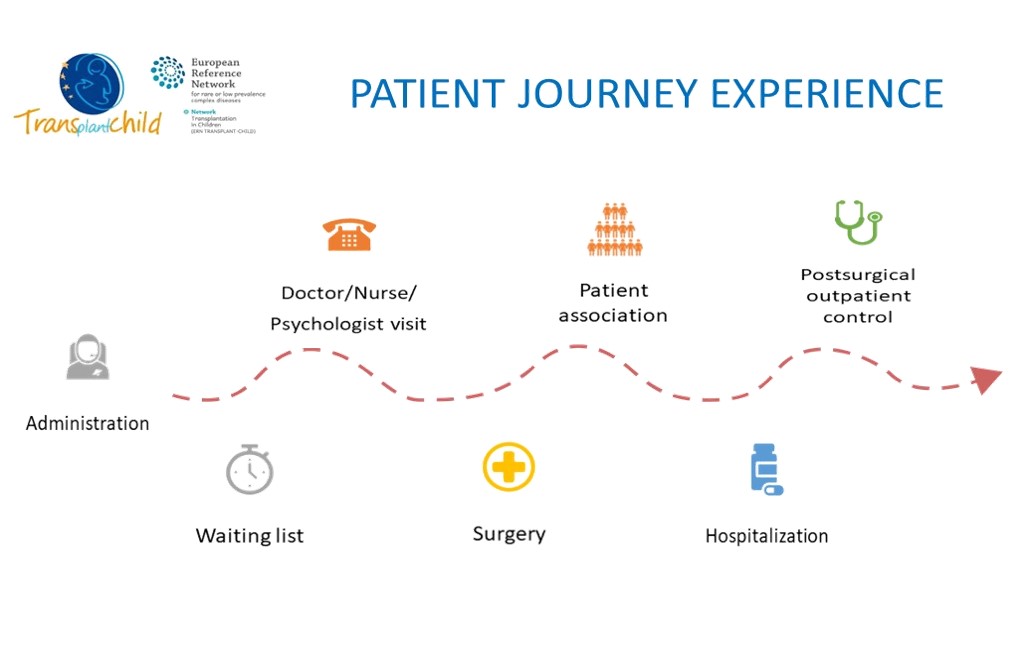
We will identify the different general indicators that are usually handled together with the areas of contact of the patient and their families with the health system, as well as professionals involved in each area, from primary care to specialized care and hospitalization. We will generate a first draft that will be proposed to the stakeholders at the meetings that will be held for this purpose as shown:
This draft will be discussed with each stakeholder group to consolidate indicators that measure patient experience in each area of interest. These interest groups will be:
- Patients
- Medical doctors, Surgeons and Psychologists
- Nurses
- Social Workers
- Clerical workers
PHASE 2
After working with stakeholders, we will go on to identify validated tools published in the scientific literature to collect the consolidated indicators
PHASE 3
Once we have the information from consensus (phase 1) and systematic review (phase 2), we will proceed to synthesize the results and design a tool proposal
PHASE 4
The proposal will be taken back to stakeholders (Figure 4) and patients will work to reach consensus and be able to design a definitive tool
PHASE 5
Subsequently, the tool must be implemented in two formats so that patients can make use of it: one format to access from mobile phone or tablet (APP) and another for access through personal computers. This implementation requires the development of different versions according to the age as previously done in other experiences:
- Children between 0 and 7 : the format of this version should be such that children of 4-5 years can understand and fill it along with the families
- Children between 7 and 14 years: this format will include the peculiarities of this stretch of age: pre-teen and adolescence.
- Children from 14 to 18 years old: with a design more adapted to the beginning stage of youth.
PHASE 6
After implementation, the tool must be validated in two ways:
- Face Validity: Validation by ERN executive committee members, ERN partners and stakeholders
- By piloting the study population: This validation will be carried out at the University Hospital La Paz. For this piloting, staff with tablets will explain and teach their use to patients and their families the day they come to the consultation to make sure that they are familiar with the tool to make a good report of the information.
PROMs
Patient Reported Outcomes Measurements
Objective
The objective of this Project is to identify validated tools of transplanted pediatric patient-reported outcomes (PROM) or their families/carers. A PROM is a standard instrument that systematically captures the patient’s perspective on the symptomatology, functional status, satisfaction, adherence and quality of life of their disease. To achieve this objective, a systematic review is proposed to answer the following questions:
– What tools to collect patient-reported results are validated for use by pediatric patients transplanted from a solid organ or hematopoietic progenitors or their families/carers?
– What are the domains and psychometric properties of these tools?
– The protocol of this systematic review is pending registration in the PROSPERO database